Streamline IMP Management & Automation Within REDCap
A low-cost, high-efficiency bolt-on solution to elevate your CTIMP database workflows without the bespoke price tag
Trusted by leading research organizations.
★★★★★
About Crispnet: Connecting Research Efforts
We develop tailored CRISPNet frameworks to work alongside REDCap - including eISFs, IMP accountability database templates, and automated pharmacy dispense forms - to simplify your clinical trial data collection drug tracking and administration.
7+
Years of Trial Administration & Data Management Experience
Integrated IMP Management
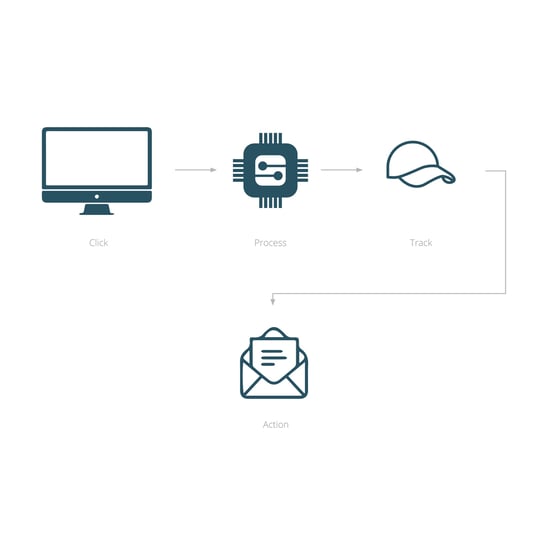
Streamlining clinical research with efficient management of investigational medicinal product supply through database automation and integration.
Avoid the Bespoke Price Tag
Connecting multiple REDCap databases ensures smooth fully auditable communication and enhanced supply chain management for clinical trials without requiring the development of a fully bespoke system.


Efficient Communication Solutions
Facilitating real-time communication between stakeholders in clinical research for informed and timely decision-making.
RED Data Dev makes advanced database automation accessible using REDCap—a tool you already know and trust.
CTIMP databases shouldn’t drain your trial budget.




With CRISPNet, you’ll get
A fully integrated system tailored to your trial
Automated IMP dispense forms ready to sign and send
eISF templates
Seamless randomisation to IMP dispense procedures
Support from a specialist with 7+ years of data mangement experience


Supply Chain
Efficient management of investigational medicinal product supply.










Crispnet has revolutionized our clinical research processes, streamlining communication and management of investigational medicinal products seamlessly. Highly recommend their innovative solutions for efficiency.
Dr. Smith

★★★★★
Communication
jdoust@crispnet.uk
+44 7388 18 3026
© 2025. All rights reserved.